Managing patients at genetic risk of breast cancer
ABSTRACTHereditary syndromes that increase the risk of breast cancer are not common, but it is critical to recognize and manage them appropriately. This paper reviews the management of patients with the most common hereditary breast cancer syndromes, ie, hereditary breast and ovarian cancer syndrome, hereditary diffuse gastric cancer, Cowden syndrome (PTEN hamartoma tumor syndrome), Peutz-Jeghers syndrome, and Li-Fraumeni syndrome.
KEY POINTS
- In addition to breast cancer, hereditary cancer syndromes increase the risk of other malignancies, with the patterns of malignancy varying by causative genetic mutation.
- Genetic counselors, medical breast specialists, surgical breast specialists, gynecologic oncologists, and others can help, but the primary care provider is the nucleus of the multidisciplinary team.
- Management of these patients often includes surveillance, chemoprevention, and prophylactic surgery.
- All decisions about surveillance, chemoprevention, and surgical risk reduction should be shared with the patient.
While most cases of breast cancer are sporadic (ie, not inherited), up to 10% are attributable to single-gene hereditary cancer syndromes.1–4 People with these syndromes have a lifetime risk of breast cancer much higher than in the general population, and the cancers often occur at a much earlier age.
With genetic testing becoming more common, primary care physicians need to be familiar with the known syndromes, associated risks, and evidence-based recommendations for management. Here, we review the management of cancer risk in the most common hereditary breast cancer syndromes, ie:
- Hereditary breast and ovarian cancer syndrome5
- Hereditary diffuse gastric cancer
- Cowden syndrome (PTEN hamartoma tumor syndrome)
- Peutz-Jeghers syndrome
- Li-Fraumeni syndrome.
IT TAKES A TEAM, BUT PRIMARY CARE PHYSICIANS ARE CENTRAL
Women who have a hereditary predisposition to breast cancer face complex and emotional decisions about the best ways to manage and reduce their risks. Their management includes close clinical surveillance, chemoprevention, and surgical risk reduction.1,4
Referral to multiple subspecialists is an important component of these patients’ preventive care. They may need referrals to a cancer genetic counselor, a high-risk breast clinic, a gynecologic oncologist, and counseling services. They may also require referrals to gastroenterologists, colorectal surgeons, endocrinologists, and endocrine surgeons, depending on the syndrome identified.
Consultation with a certified genetic counselor is critical for patients harboring mutations associated with cancer risk. The National Society of Genetic Counselors maintains a directory of genetic counselors by location and practice specialty at www.nsgc.org. The counselor’s evaluation will provide patients with a detailed explanation of the cancer risks and management guidelines for their particular condition, along with offering diagnostic genetic testing if appropriate. Women with germline mutations who plan to have children should be informed about preimplantation genetic diagnosis and about fertility specialists who can perform this service if they are interested in pursuing it.6
Screening and management guidelines for hereditary breast cancer syndromes are evolving. While subspecialists may be involved in enhanced surveillance and preventive care, the primary care physician is the central player, with both a broader perspective and knowledge of the patient’s competing medical issues, risks, and preferences.
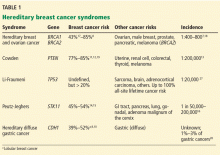
In addition to breast cancer, the risk of other malignancies is also higher, with the pattern varying by syndrome (Table 1).7–20 The management of these additional risks is beyond the scope of this review; however, primary care physicians need to be familiar with these risks to provide adequate referrals.
WHO IS AT INCREASED RISK OF BREAST CANCER?
In considering recommendations to reduce the risk of breast cancer, it is useful to think of a patient as being at either high risk or average risk.
The risk of breast cancer in women in the general population is about 12%, and most cases of breast cancer occur in patients who have no known risk factors for it. “High risk” of breast cancer generally means having more than a 20% lifetime risk (ie, before age 70) of developing the condition.
Even without a hereditary cancer syndrome, a combination of reproductive, environmental, personal, and family history factors can confer a 20% lifetime risk. But for women with hereditary syndromes, the risk far exceeds 20% regardless of such risk factors. It is likely that interactions with reproductive, environmental, and personal risk factors likely affect the individual risk of a woman with a known genetic mutation, and evidence is emerging with regard to further risk stratification.
In an earlier article in this journal, Smith and colleagues21 reviewed how to recognize heritable breast cancer syndromes. In general, referral for genetic counseling should be considered for patients and their families who have:
- Early-onset breast cancers (before age 50)
- Bilateral breast cancers at any age
- Ovarian cancers at any age
- “Triple-negative” breast cancers (ie, estrogen receptor-negative, progesterone receptor-negative, and human epidermal growth factor receptor 2-nonamplified (HER2-negative)
- Male breast cancer at any age
- Cancers affecting multiple individuals and in multiple generations.
- Breast, ovarian, pancreatic or prostate cancer in families with Ashkenazi Jewish ancestry
HEREDITARY BREAST CANCER SYNDROMES
Hereditary breast and ovarian cancer syndrome
The most common of these syndromes is hereditary breast and ovarian cancer syndrome, caused by germline mutations in the tumor-suppressor genes BRCA1 or BRCA2.7 The estimated prevalence of BRCA1 mutations is 1 in 250 to 300, and the prevalence of BRCA2 mutations is 1 in 800.1,4 However, in families of Ashkenazi Jewish ancestry, the population frequency of either a BRCA1 or BRCA2 mutation is approximately 1 in 40.1,4,6
Women with BRCA1 or BRCA2 mutations have a lifetime risk of breast cancer of up to 87%, or 5 to 7 times higher than in the general population, with the risk rising steeply beginning at age 30.1,5,8 In addition, the lifetime risk of ovarian cancer is nearly 59% in BRCA1 mutation carriers and 17% in BRCA2 mutation carriers.22
A meta-analysis found that BRCA1 mutation carriers diagnosed with cancer in one breast have a 5-year risk of developing cancer in the other breast of 15%, and BRCA2 mutation carriers have a risk of 9%.23 Overall, the risk of contralateral breast cancer is about 3% per year.3,4,24
BRCA1 mutations are strongly associated with triple-negative breast cancers.1,3,4
Hereditary diffuse gastric cancer
Hereditary diffuse gastric cancer is an autosomal-dominant syndrome associated with mutations in the CDH1 gene, although up to 75% of patients with this syndrome do not have an identifiable CDH1 mutation.9,25,26 In cases in which there is no identifiable CDH1 mutation, the diagnosis is made on the basis of the patient’s medical and family history.
Hereditary diffuse gastric cancer is associated with an increased risk of the lobular subtype of breast cancer as well as diffuse gastric cancer. The cumulative lifetime risk of breast cancer in women with CDH1 mutations is 39% to 52%,6,9–11,25 and their lifetime risk of diffuse gastric cancer is 83%.9 The combined risk of breast cancer and gastric cancer in women with this syndrome is 90% by age 80.9
Cowden syndrome (PTEN hamartoma tumor syndrome)
Cowden syndrome (PTEN hamartoma tumor syndrome) is caused by mutations in PTEN, another tumor-suppressor gene.11 The primary clinical concerns are melanoma and breast, endometrial, thyroid (follicular or papillary), colon, and renal cell cancers. Women with a PTEN mutation have a twofold greater risk of developing any type of cancer than men with a PTEN mutation.12 The cumulative lifetime risk of invasive breast cancer in women with this syndrome is 70% to 85%.11–13
Peutz-Jeghers syndrome
Peutz-Jeghers syndrome is an autosomal dominant polyposis disorder caused, in most patients, by a mutation in the serine/threonine kinase tumor-suppressor gene STK11.14
Patients with Peutz-Jeghers syndrome have higher risks of gastrointestinal, breast, gynecologic (uterine, ovarian, and cervical), pancreatic, and lung cancers. In women, the lifetime risk of breast cancer is 44% to 50% by age 70, regardless of the type of mutation.6,14,15 Breast cancers associated with Peutz-Jeghers syndrome are usually ductal, and the mean age at diagnosis is 37 years.16
Li-Fraumeni syndrome
Li-Fraumeni syndrome is an autosomal-dominant disorder caused by germline mutations in the TP53 gene, which codes for a transcription factor associated with cell proliferation and apoptosis.27
These mutations confer a lifetime cancer risk of 93% in women (mainly breast cancer) and 68% in men.1,27 Other cancers associated with TP53 mutations include sarcomas, brain cancer, leukemia, and adrenocortical tumors. Germline TP53 mutations are responsible for approximately 1% of all breast cancers.1,4
Breast cancers can occur at a young age in patients with a TP53 mutation. Women with TP53 mutations are 18 times more likely to develop breast cancer before age 45 compared with the general population.4
It is important to consider a TP53 mutation in premenopausal women or women less than 30 years of age with breast cancer who have no mutations in BRCA1 and BRCA2.1
MANAGING PATIENTS WITH GENETIC PREDISPOSITION TO BREAST CANCER
Management for patients at high risk fall into three broad categories: clinical surveillance, chemoprevention, and surgical risk reduction. The utility and benefit of each depend to a large degree on the patient’s specific mutation, family history, and comorbidities. Decisions must be shared with the patient.
CLOSE CLINICAL SURVEILLANCE
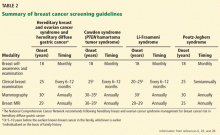
Consensus guidelines for cancer screening in the syndromes described here are available from the National Comprehensive Cancer Network at www.nccn.org and are summarized in Table 2.26,28 While the guidelines are broadly applicable to all women with these conditions, some individualization is required based on personal and family medical history.
In general, screening begins at the ages listed in Table 2 or 10 years earlier than the age at which cancer developed in the first affected relative, whichever is earlier. However, screening decisions are shared with the patient and are sometimes affected by significant out-of-pocket costs for the patient and anxiety resulting from the test or subsequent test findings, which must all be considered.
Breast self-awareness and clinical breast examination
Although controversial in the general population, breast self-examination is recommended for patients carrying mutations that increase risk.6
A discussion about breast self-awareness is recommended for all women at the age of 18. It should include the signs and symptoms of breast cancer, what feels “normal” to the patient, and what is known about modifiable risk factors for breast cancer. The patient should also be told to report any changes in her personal or family history.
Clinical breast examinations should be done every 6 months, as some cancers are found clinically, particularly in young women with dense tissue, and confirmed by diagnostic imaging and targeted ultrasonography.