Interpreting SPRINT: How low should you go?
ABSTRACTThe Systolic Blood Pressure Intervention Trial (SPRINT) found evidence of cardiovascular benefit with intensive lowering of systolic blood pressure (goal < 120 mm Hg) compared with the currently recommended goal (< 140 mm Hg) in older patients with cardiovascular risk but without diabetes or stroke. This article reviews the trial design and protocol, summarizes the results, and briefly discusses the implications of these results.
KEY POINTS
- SPRINT is the first large prospective randomized trial to show evidence of cardiovascular and mortality benefit for intensive lowering of systolic blood pressure (goal < 120 mm Hg) in older patients at cardiovascular risk, but without a history of diabetes mellitus or stroke.
- A similar trial in patients with type 2 diabetes mellitus did not show significant benefit of intensive treatment.
- Intensive treatment was associated with more adverse events, including hypotension, syncope, electrolyte abnormalities, and acute kidney injury.
- It is unclear if these results can be extrapolated to patients with a history of diabetes or stroke, younger patients, or those with low cardiovascular risk.
- Healthcare providers should engage patients in a shared decision-making process, with discussion of the benefits and risks associated with intensive lowering of blood pressure.
STUDY RESULTS
Older patients at risk, but without diabetes
Of 14,692 participants screened, 9,361 were enrolled in the study between 2010 and 2013. Baseline characteristics were comparable in both groups.
Demographics. The mean age of the participants was 67.9, and about 28% were 75 or older. About 36% were women, 58% white, 30% black, and 11% Hispanic.
Cardiovascular risk. The mean Framingham risk score was 20% (ie, they had a 20% risk of having a cardiovascular event within 10 years), and 61% of the participants had a risk score of at least 15%. Twenty percent already had cardiovascular disease.
Blood pressure. The average baseline blood pressure was 139.7/78.2 mm Hg. One-third of the participants had baseline systolic pressures of 132 mm Hg or less, another third had pressures in the range of 132 to 145, and the rest had 145 mm Hg or higher.
Renal function. The mean serum creatinine level was about 1.1 mg/dL. The mean eGFR was about 71 mL/min/1.73 m2 as calculated by the MDRD equation, and about 28% had eGFRs less than 60. The mean ratio of urinary albumin to creatinine was 44.1 mg/g in the intensive treatment group and 41.1 in the standard treatment group.
Other. The mean total cholesterol level was 190 mg/dL, fasting plasma glucose 99 mg/dL, and body mass index nearly 30 kg/m2.
Blood pressure during treatment
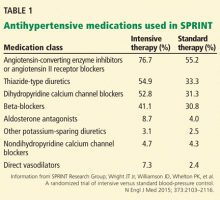
People in the intensive treatment group were taking a mean of 2.8 antihypertensive medications, and those in the standard treatment group were taking 1.8. Patients in the intensive group required greater use of all classes of medications to achieve goal systolic pressure (Table 1).
Study halted early due to efficacy
Throughout the 3.26 years of follow-up, the average difference in systolic pressure between the two groups was 13.1 mm Hg, with a mean systolic pressure of 121.5 mm Hg in the intensive treatment group and 134.6 mm Hg in the standard treatment group. The mean diastolic blood pressure was 68.7 mm Hg in the intensive treatment group and 76.3 mm Hg in the standard treatment group.
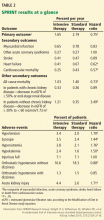
Although the study was planned to run for an average follow-up of 5 years, the National Heart, Lung, and Blood Institute terminated it early at a median of 3.26 years in view of lower rates of the primary outcome and of heart failure and death in the intensive treatment group (Table 2).
The effects on the primary outcome and mortality were consistent across the prespecified subgroups of age (< 75 vs ≥ 75), sex (female vs male), race (black vs nonblack), cardiovascular disease (presence or absence at baseline), prior chronic kidney disease (presence or absence at baseline), and across blood pressure tertiles (≤ 132 mm Hg, > 132 to < 145 mm Hg, ≥ 145 mm Hg).
Follow-up for assessment of cognitive outcomes (SPRINT MIND) and small-vessel ischemic disease (SPRINT MIND MRI) is ongoing.
WHAT DOES THIS MEAN?
SPRINT is the first large, adequately powered, randomized trial to demonstrate cardiovascular and mortality benefit from lowering the systolic blood pressure (goal < 120 mm Hg) in older patients at cardiovascular risk but without a history of diabetes mellitus or stroke.1
Most SPRINT patients had reasonably controlled blood pressure at baseline (the mean systolic pressure was 139.7 mm Hg, and two-thirds of participants had systolic pressure < 145 mm Hg). Of note, however, this trial excluded patients with systolic pressure higher than 180 mm Hg. There was excellent separation of systolic pressure between the two groups beginning at 1 year, which was consistent through the course of the trial.
The cardiovascular benefit in the intensive treatment group was predominantly driven by lower rates of heart failure (a 38% reduction in the intensive treatment group, P = .0002) and cardiovascular mortality (a 43% reduction in the intensive treatment group, P = .005), while there was no significant difference between the two groups in myocardial infarction or stroke. The beneficial effect on heart failure events is consistent with results from other trials including the Systolic Hypertension in the Elderly Program,7 Systolic Hypertension in Europe,8 and Hypertension in the Very Elderly Trial,9 all of which showed greatest risk reduction for heart failure events with systolic pressure-lowering (although to higher systolic levels than SPRINT).7–9 It is unclear why there was no beneficial effect on stroke events. The reduction in all-cause mortality in the intensive treatment group in SPRINT was greater than the reduction in cardiovascular deaths, which is also unexplained.
Although the study was terminated early due to efficacy (which introduces the possible bias that the estimated effect size will be too high), the number of primary end points reached was large (562 in the two groups combined), providing reassurance that the findings are valid. There was no blinding in the study (both participants and study investigators were aware of treatment assignment and study medications), but there was a structured assessment of outcomes and adverse events, with adjudication done by blinded reviewers.
SPRINT used an automated device for blood pressure measurement, which is known to reduce the “white coat” effect and correlates tightly with average daytime blood pressure done by ambulatory blood pressure monitoring.18 However, in clinical practice automated devices may not be available and a strict protocol for correct measurement may not be followed, with the possible result that blood pressure may be overestimated and overtreated.
What about diastolic pressure?
The trial, by design, focused on lowering systolic pressure (given the greater prevalence of isolated systolic hypertension with age), and the implications of lowering diastolic pressure are unclear. The issue of a J-shaped relationship between diastolic pressure and cardiovascular risk is debated in the literature: patients with a diastolic pressure of 60 to 65 mm Hg, especially those with existing coronary artery disease, may not tolerate aggressive blood pressure-lowering.19,20 Further analysis of this association (if any) from SPRINT will be helpful.