Biomarkers: Their potential in the diagnosis and treatment of heart failure
ABSTRACTThe increasing use of cardiac biomarkers in the diagnosis and management of heart failure (HF) has led to their inclusion in clinical practice guidelines. Studies have demonstrated that natriuretic peptides and cardiac troponins are useful adjuncts in identifying patients with HF at high risk, and we now know that a number of factors influence biomarker levels, including age, renal failure, obesity, and comorbid conditions, and that these factors as well as biomarker assay variability need to be considered when interpreting the results of biomarker testing. The broader use of cardiac biomarker testing has been limited by the lack of consistent data to support a benefit of their use in triaging management decisions, and the majority of drug therapies and titration schedules for HF were developed prior to the availability of biomarkers. Nevertheless, natriuretic peptide testing has been widely adopted, with recent guidelines supporting its use in the diagnosis of acute HF, especially in the setting of clinical uncertainty, as well as in assessing disease severity and prognosis. This review summarizes the data on traditional cardiac biomarkers and describes how the latest investigations have shaped the recommendations in the latest clinical practice guidelines.
KEY POINTS
- The usefulness of a biomarker may differ from one patient population to another, from one clinician to another, or from one clinical scenario to another.
- For risk stratification in heart failure (HF), biomarkers that reflect renal insufficiency are especially powerful prognosticators.
- In the latest clinical guidelines, natriuretic peptide testing has gained the highest level of recommendation for clinical use for any biomarker in HF.
- In general, point-of-care assays are often more variable than the same tests done in clinical laboratories; sample collection, handling, and processing also introduce variability.
GUIDELINE RECOMMENDATIONS FOR CARDIAC BIOMARKERS IN HEART FAILURE
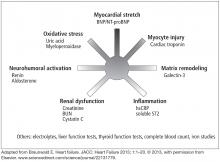
Clinical guidelines from several countries on the management of HF have expanded the role of biomarker testing in patients with HF.2,14–16 Table 1 shows the recommendations for biomarker testing in HF from the most recent joint guidelines of the American College of Cardiology and the American Heart Association. These recommendations will form the basis of the following discussion of clinically available biomarkers of HF that reflect distinct pathophysiologic processes and that have been cleared by the US Food and Drug Administration (Figure 1).
Biomarkers of myocardial stress: Natriuretic peptides
Natriuretic peptides are primary counterregulatory hormones produced in response to myocardial stress. Natriuretic peptide receptors stimulated by B-type (also “brain”) natriuretic peptide (BNP) lead to an increase in natriuresis, vasodilation, and opposing effects of other overactive neurohormonal systems. The contemporary understanding of how natriuretic peptides are being produced and metabolized is beyond the scope of this review, but generally it is now recognized that natriuretic peptide levels vary widely among patients with the same degree of symptoms or echocardiographic features.17
Of the several types of natriuretic peptide detectable by immunoassay, the two main types available for clinical use in the United States are BNP and amino acid N-terminal pro-BNP (NT-proBNP). Although there is no direct conversion available (NT-proBNP levels are five to eight times higher than BNP levels), their levels are often concordant and both are influenced by factors such as age, body mass index, and renal function. Specifically, natriuretic peptide levels in morbidly obese patients range 30% to 40% lower than levels in patients who are not morbidly obese.18
Studies over the past 10 years of natriuretic peptides in the diagnosis of HF have shown that levels are invariably elevated in underlying HF, while stable (and especially low) levels often track with clinical stability. In the latest clinical guidelines, natriuretic peptide testing has gained the highest level of recommendation for clinical use for any biomarker in HF, especially in the setting of clinical uncertainty (class 1 recommendation, level of evidence A).2,16 Two common clinical scenarios are represented in this indication. When patients present with signs and symptoms suspicious of HF (shortness of breath, fluid retention, peripheral edema, evidence of central congestion), natriuretic peptide testing provides confirmation of an underlying cardiac cause of these symptoms when elevated. Conversely, when there are alternative explanations or if the presentation is subtle and there is some degree of uncertainty, testing natriuretic peptide levels helps establish the diagnosis of HF when levels are higher than the cut-off values, and levels below the cut-off have a high negative predictive value (Table 2).19,20
Meanwhile, for patients with established HF, a deviation from “stable” natriuretic peptide levels (particularly an increase of more than 30%) may represent evolving destabilization that may warrant an intensification of therapy, whereas an unchanged or reduced level may be taken as objective evidence of clinical stability or favorable response to medical therapy. Table 3 outlines the latest Canadian guidelines that offer a practical approach as ongoing studies attempt to clarify the benefits of these strategies.15
The consistent association between elevated natriuretic peptide levels and worse prognosis21 has led to the promise that intensification of medical therapy in those with elevated natriuretic peptide levels can lead to better outcomes. Nevertheless, the rise in natriuretic peptide levels requires interpretation in the clinical context, as not all factors affecting the levels can be relieved by intensifying medical therapy (eg, age, renal insufficiency).
Several prospective, randomized controlled trials have tested this hypothesis, with favorable yet mixed results. Most studies have utilized a BNP measurement less than 100 pg/mL or an NT-proBNP measurement less than 1,000 pg/mL as a therapeutic target. In a recent prospective study that utilized the NT-proBNP threshold, only about half of patients were able to reach the target of less than 1,000 pg/mL.22 Often overlooked is the fact that in the same study, the inability to reach less than 5,000 pg/mL within 3 months after discharge clearly identified advanced, “nonresponsive” HF refractory to medical therapy and with a poor prognosis.23 This is an important point when assessing the clinical utility of biomarkers, as incremental prognostic values may not guarantee the feasibility or ultimate benefit of intensifying drug therapy according to specific biomarker targets. Until we have more insight into whether a care pathway guided by NT-proBNP measurements can lead to a consistent reduction in rates of hospitalization and mortality in HF, it is reasonable to target those with elevated natriuretic peptide levels by reevaluating their treatment regimen to achieve optimal dosing of guideline-directed medical therapy (Class 2a recommendation, level of evidence B).2 Also, the usefulness of BNP and NT-proBNP in guiding therapy for acutely decompensated HF is not well established (Class 2b recommendation, level of evidence C).2
Biomarkers of myocardial injury: Cardiac troponin
Whereas detecting circulating cardiac troponin is helpful in the diagnosis of acute coronary syndrome, the role of cardiac troponin levels in HF is primarily for risk stratification (Class 1 recommendation, level of evidence A in both acute and chronic HF).2 In patients hospitalized with acute decompensated HF, those with elevated troponin I or troponin T at the time of admission had lower systolic blood pressures, lower ejection fractions, and higher rate of in-hospital mortality.24,25 In chronic HF, elevations in both standard and high-sensitivity cardiac troponin levels were associated with increases in all-cause mortality,26 and rise in serial measurements appeared to correlate with an increased risk of future cardiovascular events.27 And with regard to cardiotoxicity, an increase in cardiac troponin over time (either after chemotherapy or with amyloidosis) is indicative of progressive cardiac dysfunction.28,29
Nevertheless, how to adjust medical therapy according to a rise in cardiac troponin levels remains unclear, as levels of cardiac troponin beyond the setting of acute coronary syndrome have appeared not to fluctuate significantly over time and do not seem to be related to underlying coronary events. Newer-generation cardiac troponin assays have yet to provide incremental value compared with standard clinical troponin assays despite their higher sensitivities.26
One common and underappreciated clinical application that combines both diagnostic and prognostic properties of both natriuretic peptide and cardiac troponin testing is the concept of HF staging. This is particularly relevant when there is a progressive change in clinical status (eg, need for hospitalization, change in signs or symptoms) or when a new therapy is started that may promote adverse effects. For example, a patient with pre-existing HF hospitalized with atypical symptoms and deemed not to have HF could be found to have subclinical myocardial necrosis as detected by low concentration of cardiac troponin or higher-than-baseline natriuretic peptide levels in the absence of hypervolemia. Careful assessment of the potential triggers of fluctuations from previous stable levels of cardiac biomarkers is also warranted (eg, atrial fibrillation, dietary indiscretion, infection, and ischemia). Indeed, these may represent objective rather than subjective changes in clinical manifestation of HF, which may warrant a reassessment of disease severity (eg, objective testing for functional capacity or hemodynamics, or even referral for consideration of advanced HF therapeutic options).