Asymptomatic carotid artery disease: A personalized approach to management
ABSTRACTAsymptomatic carotid artery disease is relatively common and poses a challenge for internists as well as vascular specialists when deciding whether to pursue surgical endarterectomy, percutaneous stenting, or medical therapy alone. The authors review the management of asymptomatic carotid disease, reflecting the most current data.
KEY POINTS
- Current guidelines are based on outdated data that may not represent the best evidence regarding the management of asymptomatic carotid disease.
- Stroke is a devastating outcome of carotid disease, and most patients and physicians are wary of deferring revascularization until a stroke occurs.
- Given the inherent risk associated with revascularization (endarterectomy or stenting) and the paucity of data, the approach should be personalized on the basis of life expectancy, sex, risk factors for stroke, and clinical acumen.
- Future research should focus on noninvasive tools to determine which patients are at high risk of stroke and may benefit from revascularization.
Flaws in the landmark trials
Beyond the debate of the questionable benefit of revascularization, well-defined flaws in the landmark trials weaken or limit their influence on current treatment guidelines and protocols for deciding whether to revascularize.
No significant benefit was found for patients over age 75.2,3 This was thought to be due to decreased life expectancy, since the benefit from revascularization becomes significant after 3 years from intervention.1–3 Also, studies have shown that increasing age is associated with a higher risk of perioperative stroke and death.20,21
Women showed no benefit at 5 years and only a trend toward benefit at 10 years (P = .05),2 likely from a higher rate of periprocedural strokes.
Blacks and Hispanics were underrepresented in the landmark studies,19 while one observational study reported a higher incidence of in-hospital stroke after carotid endarterectomy in black patients (6.6%) than in white patients (2%).29
When associated with contralateral carotid occlusion, carotid endarterectomy carries a higher risk of perioperative stroke or death.23,30,31
Carotid revascularization failed to reduce the risk of death—the total number of deaths within 10 years was not significantly reduced by immediate carotid endarterectomy compared with deferring the procedure.2
EVIDENCE SUPPORTING OPTIMAL MEDICAL MANAGEMENT
Optimal medical therapy mainly consists of antiplatelet therapy, blood pressure management, diabetic glycemic control, and statin therapy along with lifestyle changes including smoking cessation, exercise, and weight loss (Table 3).9 Detailed recommendations are provided in the American Heart Association/American Stroke Association guidelines for primary prevention of stroke.32
Antiplatelet therapy has been shown to reduce the incidence of stroke by 25%. There is no added benefit in combining antiplatelet agents unless the patient has concomitant symptomatic coronary artery disease, recent coronary stenting, or severe peripheral artery disease.33,34
Blood pressure control can reduce the incidence of stroke by 30% to 40%, and recent data suggest that drugs working on the renin-angiotensin system offer more benefit than beta-blockers for the same reduction in blood pressure.34,35
Diabetic glycemic control is supported, as higher hemoglobin A1c and fasting glucose values are associated with higher relative risk of stroke.32,36,37 However, the stroke rate does not differ significantly between patients receiving intensive therapy and those receiving standard therapy.34
Statins actually shrink carotid plaques and reduce the risk of stroke by 15% for each 10% reduction in low-density lipoprotein cholesterol. It is estimated that statin therapy confers a 30% relative risk reduction of stroke over 20 years.34,38–41
Smoking increases the overall risk of stroke by 150%, making its cessation mandatory.42
HIGH-RISK FEATURES FOR STROKE IN ASYMPTOMATIC CAROTID STENOSIS
Studies have tried to identify risk factors for stroke, so that patients at high risk could undergo revascularization and benefit from it. However, no well-defined high-risk features have yet been described that would identify patients who would benefit from early surgery.
For instance, no correlation has been found between age, sex, diabetes mellitus, lipid levels, or smoking and progression of disease.43 In contrast, having either contralateral symptomatic carotid disease or contralateral total occlusion translated into a higher ipsilateral stroke risk.18 And in several studies, the 5-year risk of ipsilateral stroke was as high as 16.2% for those with 60% to 99% stenosis.1,2,18,24,43
Features of the plaque itself
More recently, there has been a focus on plaque evaluation to predict outcomes.
Percent stenosis. An increased risk of death or stroke has been reported with higher degrees of stenosis or plaque progression.44,45 The gross annual risk of ipsilateral stroke increases from 1.5% with stenosis of 60% to 70%, to 4.2% with stenosis of 71% to 90%, and to 7% with stenosis of 91% to 99%. Nevertheless, current data are insufficient to determine whether there is increasing benefit from surgery with increasing degree of stenosis in asymptomatic carotid disease.1,3,24,44
Plaque progression translates to a 7.2% absolute increase in the incidence of stroke (1.1% if the plaque is stable vs 8.3% if the plaque is progressing). Interestingly, plaque progression to greater than 80% stenosis results in worse outcomes (relative risk 3.4, 95% CI 1.5–7.8) compared with the same level of stenosis without recent progression.33
Intimal wall thickening of more than 1.15 mm confers a hazard ratio for stroke of 3 (95% CI 1.48–6.11).46
Increased echolucency also confers a hazard ratio for stroke of 3 (95% CI 1.4–8.0).46
A low gray-scale median (a surrogate of plaque composition) and plaque area have been identified as independent predictors of ipsilateral events.44
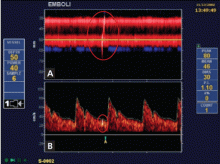
Embolic signals on transcranial Doppler ultrasonography (Figure 1) have been associated with a hazard ratio for stroke of 2.54 over 2 years.47
Carotid plaques predominantly composed of lipid-rich necrotic cores carry a higher risk of stroke (hazard ratio 7.2, 95% CI 1.12–46.20).48
High tensile stress (circumferential wall tension divided by the intima-media thickness), and fibrous cap thickening (< 500 µm) predict plaque rupture.49
Plaque ulceration. The risk of stroke increases with worsening degree of plaque ulceration: 0.4% per year for type A ulcerated plaques (small minimal excavations) compared with 12.5% for type B (large obvious excavations) and type C (multiple cavities or cavernous).50
Low cerebrovascular reactivity. Perfusion studies such as cerebrovascular reactivity evaluate changes in cerebral blood flow in response to a stimulus such as inhaled carbon dioxide, breath-holding, or acetazolamide. This may provide a useful index of cerebral vascular function. For instance, low reactivity has been associated with ipsilateral ischemic events (odds ratio 14.4, 95% CI 2.63–78.74, P = .0021).51,52 Silvestrini et al53 reported that the incidence of ipsilateral cerebrovascular ischemic events was 4.1% per year in patients who had normal cerebral vasoreactivity during breath-holding, vs 13.9% in those with low cerebral reactivity.
BEST MEDICAL THERAPY, ALONE OR COMBINED WITH REVASCULARIZATION
For carotid revascularization to be a viable option for asymptomatic carotid stenosis, the morbidity and mortality rates associated with the operation must be less than the incidence of neurologic events in patients who do not undergo the operation.54 An important caveat is that the longer a patient survives after carotid endarterectomy, the greater the potential benefit, since the adverse consequences of surgery are generally limited to the perioperative period.19
The current evidence regarding medical management of asymptomatic carotid stenosis suggests that the rate of ipsilateral stroke is now lower than it was in the control groups in the landmark trials.2,3,17,45,47,55,56 Ultimately, adherence to current best medical management takes priority over the decision to revascularize. The best current medical therapy includes, but is not limited to, antithrombotic therapy, statin therapy, blood pressure control, diabetes management, smoking cessation, and lifestyle changes (Table 3).
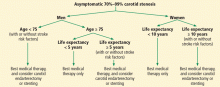
As noted above, stroke risk seems variable in the asymptomatic population according to the presence or absence of risk factors. Yet no well-defined “high-risk stroke profile” has been identified. Therefore, a patient-by-patient decision based on best available evidence should identify patients who may benefit from carotid revascularization. If asymptomatic carotid stenosis of 70% to 99% is found, factors that favor revascularization are male sex, younger age, and longer life expectancy (Figure 2).
For those with intermediate or high-risk surgical features, uncertainty exists in management since no studies have compared revascularization against medical management only in this group of patients.1 However, data from high-risk cohorts had high enough complication rates in both intervention arms to question the benefit of revascularization over medical therapy.20,21 Therefore, the individual perioperative risk of stroke, myocardial infarction, and death must be weighed against the potential benefit of revascularization for each patient.
If revascularization is pursued, studies have demonstrated that carotid artery stenting is not inferior to endarterectomy15,16 in high-surgical-risk patients. However, the revascularization approach must be tailored to the patient profile, since stenting demonstrated a lower risk of periprocedural myocardial infarction but a higher risk of stroke compared with endarteretomy.20
Finally, the current acceptable risks of perioperative stroke and death must be revised if revascularization is elected. Current data suggest that a lower threshold—around 1.4%—can be used.20 Moreover, further guidelines must determine the impact of adding myocardial infarction to the tolerable perioperative risks, since it has been excluded from main trials and guidelines.20