GERD: Diagnosing and treating the burn
ABSTRACTGastroesophageal reflux disease (GERD) is chronic, very common, and frequently encountered in internal medicine and subspecialty clinics. It is often diagnosed on clinical grounds, but specialized testing such as endoscopy and pH monitoring may be necessary in certain patients. Although proton pump inhibitors (PPIs) are the mainstay of treatment, clinicians should be aware of their short-term and long-term side effects.
KEY POINTS
- GERD symptoms may be typical (eg, heartburn, regurgitation) or atypical (eg, cough, chest pain, hoarseness).
- In patients with typical symptoms, a 6- to 8-week trial of a PPI is a reasonable and cost-effective approach to diagnosing GERD.
- Endoscopy is indicated for patients who have alarm symptoms such as dysphagia, weight loss, and bleeding; it is unnecessary in patients who have typical GERD symptoms.
- Ambulatory pH monitoring should be used in patients whose symptoms do not respond to a PPI and those in whom antireflux surgery is being considered.
- Weight loss and head-of-bed elevation are the only lifestyle interventions that have been proven effective for GERD.
- While risks of PPI use are rare, they should be discussed with patients on long-term therapy.
- Symptoms that do not respond to a PPI are less likely to improve with antireflux surgery.
Endoscopy
Endoscopic findings in GERD can include erosive esophagitis, peptic stricture, and Barrett esophagus. Esophageal erosions are a highly specific sign of GERD; the Los Angeles classification system, a standardized scale for grading the severity of erosive esophagitis (from A to D, with D the most severe) provides an objective way to assess severity.8 However, most patients with heartburn and regurgitation do not have erosive disease, thus limiting the sensitivity of upper endoscopy as an initial diagnostic test in patients with suspected GERD.9
We recommend endoscopy for patients who present with alarm symptoms, patients with noncardiac chest pain, PPI nonresponders, and patients with chronic GERD symptoms and multiple risk factors for Barrett esophagus besides GERD, such as older age, male sex, white race, overweight, and smoking.10
Ambulatory pH and impedance monitoring
Ambulatory pH monitoring is the gold standard test for pathologic acid exposure in the esophagus. pH testing is indicated in PPI nonresponders, patients presenting with atypical symptoms, and before antireflux surgery.
In general, pH testing should be performed after the patient has been off PPI therapy for at least 7 days, as the test is highly unlikely to be abnormal while a patient is on a PPI.11 It is done either with a transnasal catheter for 24 hours, or with a wireless capsule (Bravo pH System, Given Imaging Ltd, Duluth, GA), which collects 48 to 96 hours of data. Studies of the wireless system have shown that its sensitivity increases 12% to 25% when it is performed for 48 hours compared with 24 hours.12,13
The pH test can be combined with impedance testing to evaluate for nonacid reflux.14 However, the clinical significance of nonacid reflux remains controversial, and for this reason the Esophageal Diagnostic Advisory Panel recommends that the decision to perform antireflux surgery should not be based on abnormal impedance testing.15
During pH and impedance testing, special software can calculate how closely the patient’s symptoms correlate with esophageal acid exposure. The symptom index (SI) and symptom association probability (SAP) are the symptom measurements most commonly used in practice. The SI measures the overall strength of the relationship, and an SI greater than 50% is considered positive.16 The SAP determines whether this relationship could have occurred by chance, and an SAP greater than 95% is statistically significant.17 In patients with normal levels of esophageal acid exposure, an elevated SI or SAP may indicate a component of esophageal hypersensitivity in symptom generation.
At our institution, we generally perform pH-only transnasal or wireless testing off PPI therapy to establish that the patient has pathologic acid exposure in the distal esophagus. Combined pH-impedance testing is typically reserved for patients with atypical symptoms unresponsive to PPI therapy and abnormal results on previous pH testing, which allows for correlation of nonacid reflux and symptoms.
Other tests
Esophageal manometry and barium esophagography have limited value in the primary diagnosis of GERD. However, they should be considered to rule out achalasia and other esophageal motility disorders in patients whose symptoms do not respond to PPIs. For this reason, esophageal manometry should be performed before considering antireflux surgery.
MANAGING GERD
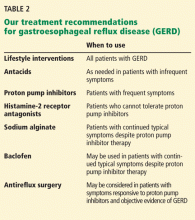
Table 2 summarizes the various treatments for GERD.
Lifestyle modifications
Lifestyle modifications are the first-line therapy for GERD. Modifications that have been studied include weight loss, head-of-bed elevation, and avoidance of tobacco, alcohol, and late-night meals. Another modification that has been suggested is avoiding foods that can aggravate reflux symptoms—eg, caffeine, coffee, chocolate, spicy foods, highly acidic foods (oranges, tomatoes), and fatty foods. Of these, only weight loss and head-of-bed elevation have been proven effective.18
Three randomized controlled trials demonstrated that GERD symptoms and esophageal pH values improved with head-of-bed elevation using blocks or incline foam wedges.19–21 Several cohort studies demonstrated reduction in GERD symptoms with weight loss.22,23 Recently, a prospective cohort study also found that smoking cessation significantly improved GERD symptoms in patients with normal body mass index and severe symptoms.24
Antacids
Several antacids (eg, sodium bicarbonate, calcium carbonate, magnesium hydroxide, aluminum hydroxide) are available over the counter.
Antacids were thought to control heartburn symptoms by increasing the pH of gastric contents that might subsequently reflux into the esophagus. However, well-controlled studies have shown that they relieve heartburn by neutralizing acid in the esophagus, with no significant effect on gastric pH.25,26
Antacids provide rapid but short-lived relief from an existing episode of heartburn. Because they do not significantly raise the gastric pH, they do not prevent subsequent reflux episodes from repeatedly exposing the esophagus to gastric acid and causing heartburn. Additionally, antacids have not been shown to contribute to healing of erosive esophagitis.27 Hence, they may not be optimal for treating frequent reflux heartburn.
Sodium alginate
Gastric acid pockets are unbuffered pools of acid that float on top of ingested food.28 They develop as a result of poor mixing of newly secreted acid and food in the proximal stomach, which remains relatively quiescent after a meal compared with the distal stomach.29 In GERD, proximal extension of the acid pocket above the diaphragm increases the risk of acid reflux.30 The acid pocket is therefore an important source of postprandial acid in GERD and, as such, represents a unique therapeutic target.
Emerging evidence suggests that alginates may act directly on the acid pocket. Alginates are natural polysaccharide polymers that, on contact with gastric acid, precipitate within minutes into a low-density viscous gel of near-neutral pH. The change in pH triggers the sodium bicarbonate in the formulation to release carbon dioxide that becomes trapped in the alginate gel, causing it to float to the top of the gastric contents like a raft.31
A randomized controlled trial demonstrated that sodium alginate was as effective as omeprazole in relieving symptoms in patients with nonerosive reflux disease.32 Alginate has also been shown to provide more postprandial reflux relief than antacids.33
Histamine-2 receptor antagonists
Histamine-2 receptor antagonists act more swiftly and increase postprandial gastric pH more rapidly than PPIs, thus making them a good option for prophylaxis against postprandial GERD.34 Taking these drugs at bedtime may help in patients with objective nighttime reflux despite optimal PPI use. However, tachyphylaxis may occur as early as 1 week after starting combination therapy.35
Proton pump inhibitors
There are currently seven available PPIs, including four that can be obtained over the counter (omeprazole, lansoprazole, esomeprazole, and omeprazole-sodium bicarbonate) and three available only by prescription (rabeprazole, pantoprazole, and dexlansoprazole). Studies have shown than a standard 6- to 8-week course of a PPI provides complete symptom relief in 70% to 80% of patients with erosive reflux disease and in 60% of patients with nonerosive reflux disease.36,37 Clinically, PPIs all appear to be similar in their symptom relief.38
Most PPIs should be taken 30 to 60 minutes before meals. Exceptions are omeprazole-sodium bicarbonate and dexlansoprazole, which can be taken without regard to meals. At our institution, we usually start PPIs in a once-daily standard dose for 6 to 8 weeks and consider increasing to twice-daily dosing if symptoms do not respond completely. Patients with mild intermittent GERD symptoms may benefit from “on-demand” use of PPIs. This approach is best suited for patients with nonerosive reflux disease without evidence of Barrett esophagus on endoscopy.
Safety and adverse effects of PPIs
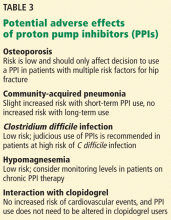
PPIs are generally safe but can cause adverse effects (Table 3).
Osteoporosis. In 2010, the US Food and Drug Administration issued warnings regarding the potential for wrist, hip, and spine fractures in PPI users.26 Most recent evidence suggests that PPIs may be associated with a small increase in risk of hip fractures in patients already at high risk.39,40 However, the 2013 American College of Gastroenterology (ACG) guidelines say that patients with known osteoporosis can remain on PPI therapy, and concern for hip fractures and osteoporosis should not affect the decision to use PPIs long-term except in patients with other risk factors for hip fracture.41
Community-acquired pneumonia. An increased risk of community-acquired pneumonia cannot be clearly documented in association with PPI therapy. Multiple studies, including randomized controlled trials, investigated this potential correlation. However, evidence suggests that short-term but not long-term PPI use may be associated with an overall increased risk of community-acquired pneumonia.42,43 Current guidelines suggest that in patients who need a PPI, the drug should not be withheld on the basis of a potential risk of community-acquired pneumonia.41
Clostridium difficile infection. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally. In fact, studies and meta-analyses have suggested that PPIs do increase the risk of development and recurrence of C difficile infection.44,45 Therefore, PPIs should be used with care in patients who are at risk.41
Interaction with clopidogrel. The antiplatelet activity of clopidogrel requires activation by CYP2C19, the same pathway required for metabolism of some PPIs. Concern was raised about decreased antiplatelet activity of clopidogrel in the presence of PPIs. This was extensively studied, and there now appears to be no increased risk of adverse cardiovascular events in patients on PPIs, based on data from well-controlled randomized trials.46,47 A consensus panel of the American College of Cardiology Foundation, the American Heart Association, and the ACG said that PPIs may be used for appropriate indications in patients taking clopidogrel.47
Hypomagnesemia. By an unknown molecular mechanism, PPIs are thought to reduce intestinal magnesium absorption, leading to hypomagnesemia. A meta-analysis published in 2011 showed that PPI-induced hypomagnesemia is a drug-class effect and occurred after a median of 5.5 years of PPI use. Stopping the PPI resulted in magnesium recovery in 4 days, and rechallenge led to recurrence within 4 days.48
Hence, to avoid putting patients on long-term PPI therapy at risk, clinicians should anticipate this problem. Our practice is to check the magnesium level before starting a patient on long-term PPI therapy, and then to repeat the measurement every 1 to 2 years.