Assessing Attributes of Topical Vehicles for the Treatment of Acne, Atopic Dermatitis, and Plaque Psoriasis
There is limited information available regarding patient preferences and attributes of topical product formulations for specific dermatologic conditions. This study focused on product attributes that were most desirable for 3 dermatologic conditions: acne, atopic dermatitis (AD), and plaque psoriasis (PP). Six focus groups were conducted with participants self-reporting 1 of these conditions and use of 2 or more topical treatments. Discussion focused on symptoms, treatments tried, and vehicle attributes. Fifty-four subjects participated: acne, n=19; AD, n=18; and PP, n=17. The most commonly reported prescription medication vehicles were creams and ointments, followed by lotions, gels, and foams. Itching and redness were the only symptoms spontaneously reported across all 6 focus groups. The attributes considered most important across all conditions included: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferences attributable to acne included: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container is not easily broken/does not leak, and creamy. Preferences attributable to AD included: not noticeable to others/conceals area, good consistency, and cooling. Patient preference for product vehicle is relevant to adherence as compliance is a major factor for high rates of failure for dermatologic treatments.
- Patient preference for topical product formulation varies by dermatologic condition being treated.
- Most important vehicle attributes cited across study conditions include moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
- Patient preference for product vehicle is relevant to treatment adherence and may vary depending on the condition being treated.
Results by Condition
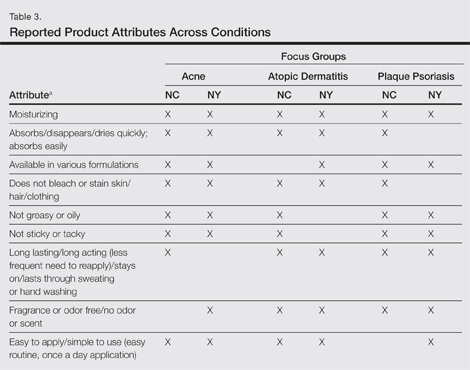
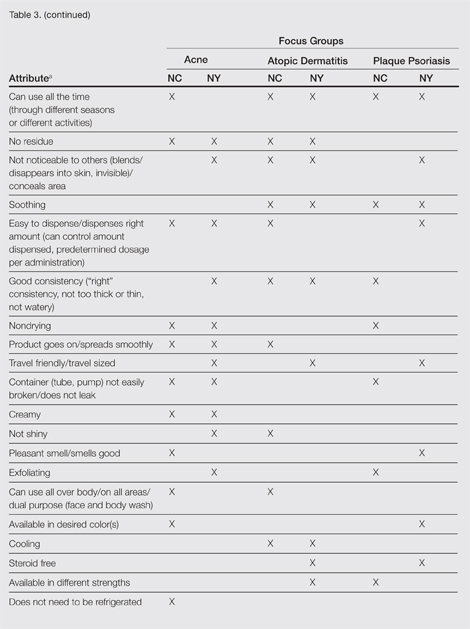
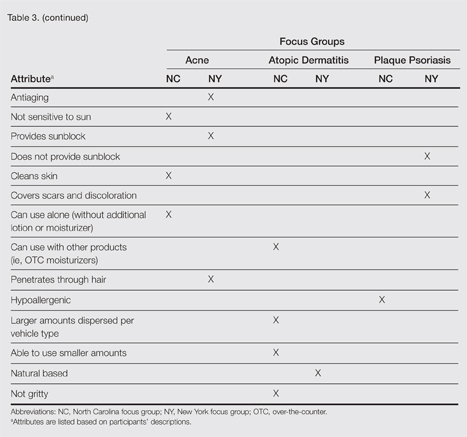
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.