Targeted therapies forge ahead in multiple breast cancer subtypes
Citation JCSO 2017;15(5):e277-e282
©2017 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0372
Submit a paper here
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
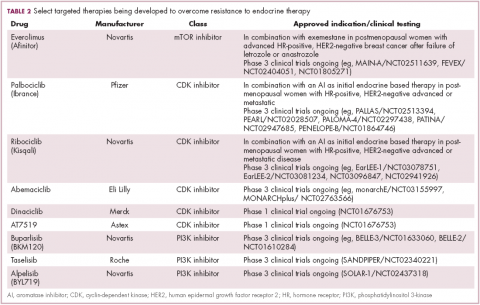
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
,Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.