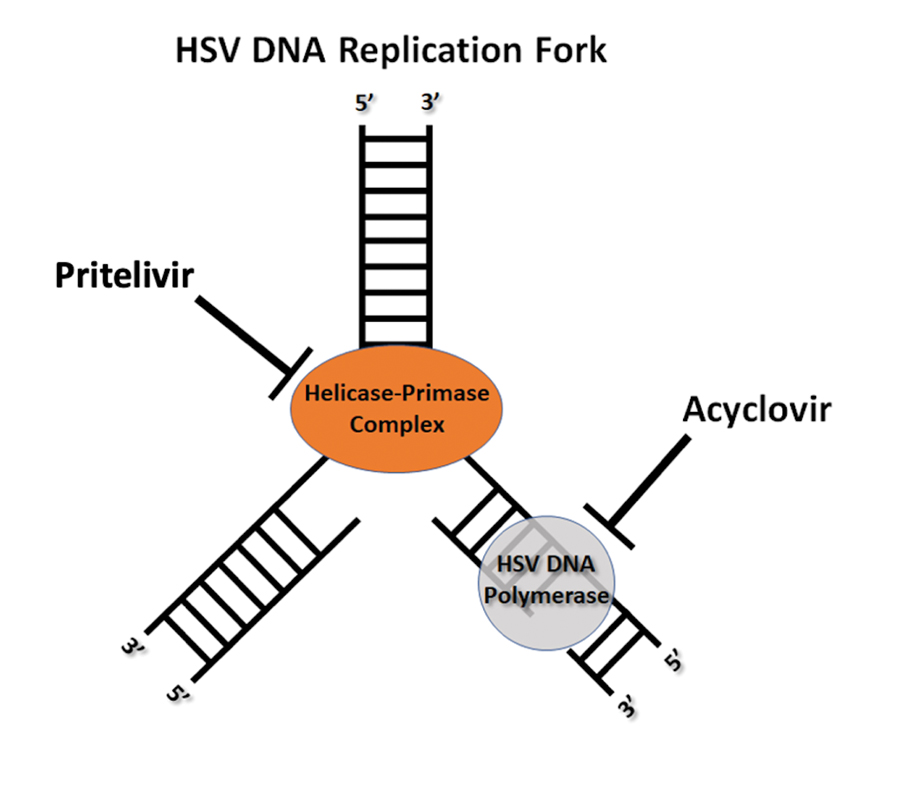
Painful Edematous Labial Erosions
THE DIAGNOSIS: Kaposi Varicelliform Eruption
Genital erosions tested positive for herpes simplex virus (HSV) 2 via PCR, confirming a Kaposi varicelliform eruption (KVE) in a patient with mycosis fungoides. The medical team began antiviral therapy with intravenous (IV) acyclovir; however, susceptibility testing during the hospital admission confirmed acyclovir resistance, requiring a transition to cidofovir cream 1% and IV foscarnet.1 Subsequent concerns by the care team about chemical burns, dysuria, and renal impairment led to discontinuation of both the cidofovir and foscarnet, considerably narrowing the treatment options.1 The patient’s condition was complicated by polymicrobial bacteremia. Additionally, worsening acidosis and acute kidney injury required initiation of continuous renal replacement therapy.1 Considering these conditions, the patient was enrolled in a promising clinical trial for pritelivir, a novel antiviral medication; however, due to the development of oliguria and the progression of renal failure, this course of treatment had to be discontinued. Faced with potential viral encephalitis, the infectious disease team concluded that, despite previous adverse reactions, resumption of IV foscarnet treatment would present more benefits than risks, given the patient’s critical situation.1
Mycosis fungoides (MF) is a slowly progressive cutaneous T-cell lymphoma of CD4+ cells that primarily affects the skin. Clinically, it often is characterized by pruritic scaly patches or plaques with sharply demarcated borders, the enduring nature of which consistently poses a therapeutic challenge due to their noted resistance to preliminary lines of treatment. Presently, potential cures are limited to allogeneic stem cell transplantation and unilesional radiotherapy for advanced MF; however, no treatment has been found to notably improve survival rates.1 Mycosis fungoides can result in various complications including diffuse spread of a skin infection caused by HSV, known as KVE.1 Kaposi varicelliform eruption usually manifests clinically with painful skin vesicles that often are accompanied by systemic signs such as fever and malaise. The vesicles rapidly progress into pustules or erosions, predominantly affecting regions such as the head, neck, groin, and upper torso (Figure 1).2 Kaposi varicelliform eruption is considered a dermatologic emergency due to its potential to precipitate serious complications such as life-threatening secondary bacterial infection, HSV viremia, and multiorgan involvement; it also carries the risk of instigating ocular complications, such as keratitis, conjunctivitis, blepharitis, uveitis, and potential vision loss.2
Kaposi varicelliform eruption usually is diagnosed through clinical examination supported by polymerase chain reaction, viral culture, histopathology, HSV serology, and Tzanck smear.2 The differential diagnosis includes varicella, atypical varicella, herpes genitalis, herpes zoster, allergic or irritant contact dermatitis, or MF, which may result in painful skin ulcers.2-4 If an HSV superinfection is suspected, a polymerase chain reaction test ideally should be conducted within the first 72 hours of symptom onset.2 Herpes simplex virus infection may be reinforced by histologic features such as intraepidermal blistering, acantholysis, keratinocyte ballooning degeneration, and multinuclear giant cells with intranuclear inclusions. Given its severe nature, immediate empiric antiviral treatment for KVE is essential, even while awaiting confirmatory tests. The recommended treatment protocol involves acyclovir (400 mg orally 3 times daily or 10 mg/kg IV) or valacyclovir (500 mg orally twice daily), continued until KVE resolves.2
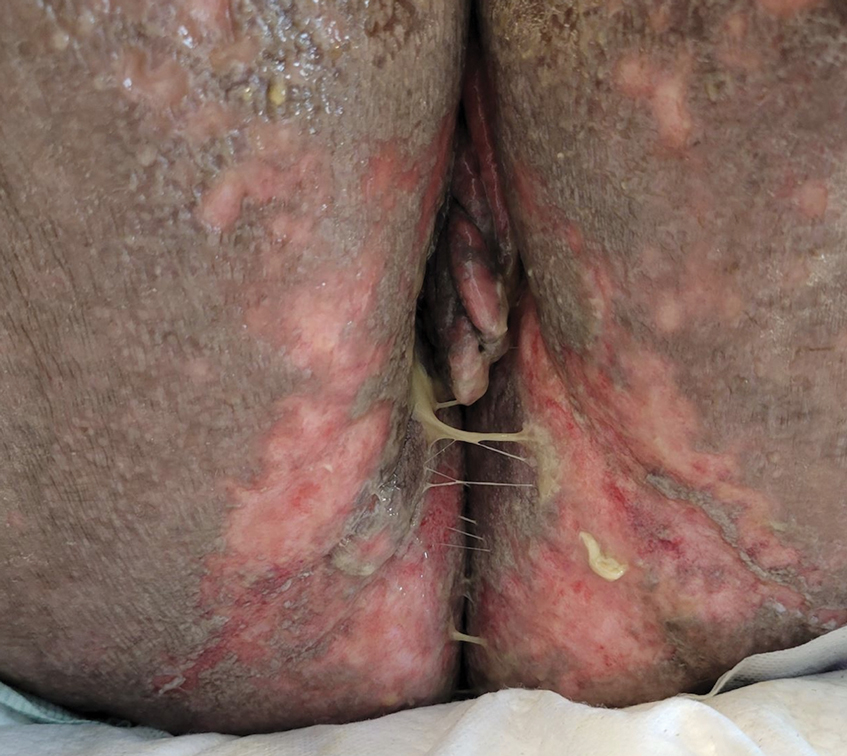
Herpes genitalis caused by HSV-2 is estimated to affect approximately 45 million adults in the United States.2 First-line treatment for HSV-2 includes acyclovir and its derivatives, which are viral nucleoside analogs that inhibit viral DNA polymerases.5,6 However, over the past 2 decades, increasing HSV resistance to acyclovir and its derivatives has been noted among immunocompromised patients.5,6 Second-line agents, such as IV foscarnet and cidofovir, require close laboratory monitoring for nephrotoxicity and are contraindicated in those with renal insufficiency, thus limiting their use.5 To combat acyclovir resistance, novel antivirals such as pritelivir are being developed. Pritelivir targets the HSV helicase-primase complex and has been shown to outperform acyclovir in in-vitro animal models.7 Due to its unique mechanism of action (Figure 2), pritelivir is effective against acyclovir-resistant HSV strains, and clinical trials suggest its serum half-life may allow for daily dosing. A phase 2 study showed pritelivir reduced viral shedding days, sped up genital lesion healing in adults infected with HSV-2, and exhibited a good safety profile.7 Our patient participated in ongoing open-label trials of pritelivir that aimed to assess its efficacy and safety in immunocompromised patients. Given the limited alternative treatments for acyclovir-resistant HSV-2, clinicians need to stay updated on antiviral agents under development.