Heart rate variability with deep breathing as a clinical test of cardiovagal function
ABSTRACT
Research into heart rate variability (HRV) and respiration over the past 150 years has led to the insight that HRV with deep breathing (HRVdb) is a highly sensitive measure of cardiovagal or parasympathetic cardiac function. This sensitivity makes HRVdb an important part of the battery of cardiovascular autonomic function tests used in clinical autonomic laboratories. HRVdb is a reliable and sensitive clinical test for early detection of cardiovagal dysfunction in a wide range of autonomic disorders.
Heart rate variability (HRV) has been a focus of interest in cardiovascular physiology for more than 150 years. This review will briefly survey the history of research linking HRV to respiration and then explore the clinical significance of this linkage, with a focus on HRV with deep breathing.
HRV AND RESPIRATION: THE EARLY RESEARCH
The first report linking HRV to respiration has been credited to Karl Ludwig, who in 1847 noted that heart rate increased with inspiration and decreased with expiration.1,2 The precise origin of this variability has been studied extensively, but a single unifying mechanism defining the determinants of HRV with respiration has not been established. However, several mechanisms have been identified that may be contributing to HRV. Hering in 1871 noted in dog experiments that inflation of the lungs was associated with a tachycardia and that additional higher-pressure insufflation resulted in a bradycardia. He concluded that HRV was determined by pulmonary reflexes.2,3 Bainbridge observed in dog experiments in 1915 that the heart rate increased during the diastolic filling of the heart that occurred during inspiration.4 In a subsequent article, published in 1920, Bainbridge attributed HRV to this reflex, which now carries his name.5
There is also evidence that HRV may be caused by central nervous system mechanisms. Canine experiments have revealed that rhythmic variations in the heart rate and ventricular pressure waves may coincide with rib cage movements in innervated, isovolumetric, left ventricular preparations.6 These data are consistent with radiation of respiratory center activity to the cardiovascular autonomic centers in the medulla resulting in HRV. There is also evidence that stretch of the right atrium and sinus node region may produce HRV via cardiac reflexes.7 It is likely that all of these mechanisms are contributing at some level to the HRV that is observed with respiration.
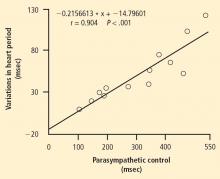
INSIGHTS INTO CLINICAL IMPLICATIONS
METHODS OF MEASURING HRV
A wide variety of methods have been developed to measure HRV.11,12 Some of the methods employ statistical analysis, typically of prolonged recordings of 24 hours or longer. These methods include simple statistics such as the standard deviation of the heart rate or the R-R interval as well as more complex statistical measures such as the mean squared successive difference of the R-R intervals. These methods have been applied mostly to the analysis of prognosis following acute myocardial infarction. Reduced HRV has been established as a powerful predictor of mortality and arrhythmic complications following acute myocardial infarction.11 The methods developed for clinical tests of cardiovagal function typically involve measuring HRVdb over short intervals (< 90 sec). Deep breathing magnifies HRV with respiration, allowing for methods to assess HRV with respiratory cycles.
FACTORS THAT AFFECT HRV WITH DEEP BREATHING
Many variables may affect HRVdb.12 HRVdb is influenced by age, as the variability decreases with advancing age, so it is essential to use methods with well-defined age-stratified normal values. HRVdb is maximal when the patient is lying supine and breathing at a rate of 5 to 6 breaths per minute. The depth of breathing for a maximum result requires a tidal volume of approximately 1.2 L for an average adult. Protocols that involve breathing for more than 90 seconds may induce hypocapnea, which can reduce HRVdb. Most importantly, numerous medications can affect HRVdb. Medications with anticholinergic activity, including over-the-counter cold medications, tricylic antidepressants, and antispasmodics, should be discontinued at least 48 hours prior to testing, if possible. Patients are also instructed to not drink caffeinated beverages, use nicotine, or drink alcohol 3 hours prior to testing.
CLINICAL APPLICATIONS
HRVdb represents a very sensitive measure of cardiovagal or parasympathetic cardiac function and thus is an important component of the battery of cardiovascular autonomic function tests used in clinical autonomic laboratories. In most autonomic disorders, parasympathetic function is affected before sympathetic function, so HRVdb provides a sensitive screening measure for parasympathetic dysfunction in many autonomic disorders. HRVdb has proven to be a sensitive and reliable clinical test for the early detection of cardiovagal dysfunction in a wide spectrum of autonomic disorders, including diabetic autonomic neuropathy,14 uremic neuropathy,17 familial autonomic neuropathies,18 and various small fiber neuropathies.19,20 HRVdb has also been valuable in assessing patients with pure autonomic failure,21 multisystem atrophy,22 and other central neurodegenerative disorders.23