Palliative Radiotherapy for the Management of Metastatic Cancer
In recent years, there has been increasing interest in palliative care for patients with cancer at the end of life. Up to 23% of patients have metastatic disease at presentation, and symptoms from metastatic lesions can cause significant anxiety and impair patients’ quality of life (QOL).1
Palliative radiotherapy (RT) plays a valuable role in the management of metastatic disease to relieve tumor-related symptoms. Although palliative RT does not provide a chance for a cure, it improves QOL and may prolong survival time.2-4 An estimated 20% to 50% of radiation courses are prescribed with palliative intent, because RT is highly effective in providing symptom relief, and the toxicity associated with palliative doses is typically mild.5,6 Palliative RT can be used to manage bone and brain metastases, prevent or treat spinal cord compression, and manage numerous tumor-related symptoms, such as pain and bleeding in patients with terminal cancer.
Palliative RT for bone and brain metastases is supported by high-quality evidence and is considered one of the most effective and cost-effective options available.7,8 This article aims to review the role of RT in treating 3 conditions commonly encountered in patients with metastatic disease—bone metastases, spinal cord compression, and brain metastases—and to emphasize the importance of timely integration of RT for optimal results.
Bone Metastases
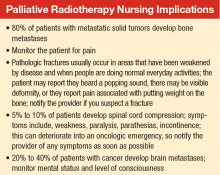
About 80% of patients with metastatic solid tumors develop bone metastases, and about 350,000 deaths are linked to bone metastases in the U.S. each year.9 Osseous
metastases can lead to pain, fracture, hypercalcemia, and spinal cord compression. The primary modality for treatment of pain and prevention of morbidity from bone metastases is external beam RT.10
The likelihood of bone pain relief with palliative RT is 60% to 80%, and 30% to 40% of patients achieving complete pain relief. Randomized studies have shown multiple-dose and fractionation regimens provided effective symptom relief for bone metastases. Most commonly used regimens include a single fraction of 8 gray (Gy) delivered in 1 treatment, 20 Gy in 5 fractions delivered daily over 1 week, and 30 Gy in 10 fractions delivered over 2 weeks. Treatment with a single fraction improves access to treatment and patient convenience, whereas more prolonged courses have been associated with lower rates of retreatment.11,12 Regarding the higher rate of retreatment with single-fraction RT, no clear evidence exists that this is due to a less durable pain response or lower level of pain relief.13
There has been recent interest in using predictive models to estimate life expectancy to avoid long courses of RT at the end of life.14,15 Shorter treatment courses of 8 Gyonce or 20 Gy in 5 fractions are particularly valuable for patients with a life expectancy < 3 months to avoid long courses of treatment, and thereby improve QOL as patients transition into hospice. A recent survey demonstrated that 93% of radiation oncologists within the VHA are willing to prescribe short courses of RT consisting of ≤ 6 fractions, and 76% have experience with single-fraction RT.16 These findings are in contradiction to the findings in the non-VA radiation oncology community, in which < 10% of patients with uncomplicated bone metastases are treated with a single fraction.17,18
In addition to providing pain relief, RT is used in the treatment of impending fractures either, adjuvant after surgical stabilization or alone for lower risk lesions.19 Factors that impact fracture risk include location of the metastasis (weight-bearing bones, such as femurs, which are at particularly high risk), length of bone involved, and extent of cortical involvement. Mirels’ scoring system was developed to predict fracture risk in patients with bone metastasis, based on 4 criteria: the
extent of cortical involvement, the location of the metastasis, the osteolytic vs osteoblastic appearance of the lesion, and the degree of pain.20 Surgical fixation can be considered, based on the total score and corresponding fracture risk. When appropriate, surgical stabilization should be considered by an orthopedic surgeon prior to initiating RT.
Postoperative RT after surgical stabilization has been associated with a reduced rate of secondary surgical procedures as well as with improved functional status.21 Radiotherapy promotes remineralization and bone healing and prevents the loss of surgical fixation by treating any residual tumor. A retrospective review of 60 patients with metastatic disease in weight-bearing bones with pathologic fracture or impending pathologic fracture demonstrated that surgery followed by RT was associated with improved functional status as well as with improved overall survival (OS).22,23 For patients in whom surgery is not indicated, the consulting radiation oncologist should consider factors such as the location of the metastasis in weight-bearing vs nonweight bearing bones, the size and extent of the metastasis, and associated symptoms when making a treatment recommendation. In patients at fracture risk from bone metastases, bisphosphonates should also be considered as part of the treatment regimen.24
Spinal Cord Compression
About 5% to 10% of patients diagnosed with cancer will develop spinal cord compression during the course of their disease.25 Spinal cord compression is considered a medical emergency that can result in significant pain and neurologic symptoms, including weakness, paralysis, parasthesias, and incontinence. Early treatment of spinal cord compression can prevent onset or progression of these symptoms; furthermore, early treatment prior to loss of ambulation is associated with improved long-term ambulatory function.26,27
Treatment decisions for spinal metastases with an associated concern for cord compression should be made after a consultation with both a neurosurgeon and a radiation oncologist. Early initiation of steroids is recommended to aid in tumor shrinkage for potential symptom relief.28 A standard way to administer dexamethasone is with a 10-mg loading dose followed by 16 mg per day, divided into 4 doses of 4 mg. Higher steroid doses showed no benefit in a prospective randomized trial comparing 96 mg with 16 mg of dexamethasone daily.29
Surgical decompression should be considered initial management of spinal cord compression. For patients treated surgically, local RT is indicated postoperatively as well. Randomized data show that surgery followed by RT provides better ambulatory function than does RT alone in patients with paralysis of < 2 days’ duration.30 Some patients with metastatic disease are not good candidates for surgery due to comorbidities, poor performance status, life expectancy < 3 months, or multilevel spinal involvement.
In patients who are not operative candidates, radiation alone is an appropriate alternative. However, several factors need consideration in deciding whether to manage cord compression with surgery followed by RT vs RT alone. These factors include life expectancy, tumor type (myeloma and lymphoma are more radiosensitive), interval since tumor diagnosis, and the presence of visceral metastases.31 Factors favoring surgical decompression plus postoperative RT over RT alone include spinal instability, KPS (Karnofsky Performance Status) > 70, radio-resistant tumor histology, minimal metastatic disease, and projected survival > 3 months.10
For patients managed with RT alone, early diagnosis and treatment is associated with improved outcomes. A prospective study of patients treated with RT without surgery for spinal cord compression demonstrated that 82% of patients experienced back pain relief, 76% achieved improvement in or preservation of ambulation, and 44% of patients with sphincter dysfunction experienced improvement with treatment.32 Patients with certain tumor histologies, such as myeloma, breast cancer, and prostate cancer, had better responses to RT.32
In the setting of spinal cord compression, longer courses of RT may provide better local control than do shorter courses.33 Therefore, longer courses of RT, such as 30 Gy in 10 fractions delivered over 2 weeks, are often preferred in cases of spinal cord compression treated with definitive RT as well as after surgical decompression. However, overall life expectancy is an important factor considered by the treating radiation oncologist when selecting a short course vs a longer course of RT.
In the instance of painful vertebral body metastases without spinal cord compression, a new subset analysis of the Radiation Therapy Oncology Group (RTOG) 9714 randomized trial indicated that single fraction RT (8 Gy) is just as effective as multiple fractions (30 Gy in 10 fractions), with this study demonstrating comparable rates of pain relief and narcotic use in both groups 3 months after RT.34 Advantages to the single-fraction plan compared with those of multiple fractions include mitigation of logistic concerns for patients and family at the end of life and less acute adverse effects.
Brain Metastases
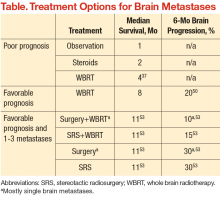
An estimated 20% to 40% of patients with cancer develop brain metastases.35 The incidence of brain metastases has been rising most likely due to improved detection rates with magnetic resonance imaging (MRI) and improved cancer survival, because treatment regimens have improved with targeted chemotherapy and radiation techniques. Currently, the annual incidence of brain metastases is 170,000 to 200,000 in the U.S.36 Prognosis for these patients is poor, with median survival of 1 month without treatment and about 4 months with whole brain RT (WBRT) (Table).25,37-39
The goal of management for patients with brain metastases is to prevent or treat neurologic symptoms and to prolong survival. Treatment options include corticosteroids, WBRT, surgery, and stereotactic radiosurgery (SRS). Recommendations for treatment should involve both a radiation oncologist and neurosurgeon to determine the best treatment for an individual based on patient age, performance status, extent of systemic disease, and number of brain metastases. These prognostic factors that may predict life expectancy and impact treatment recommendations.40
Factors that have been correlated with improved survival include younger age, better performance status, fewer brain metastases, and lower burden of systemic disease.41,42 Prognostic assessment tools such as the Graded Prognostic Assessment and RTOG-Recursive Partitioning Analysis can be used to predict life expectancy in patients with brain metastases.41,43 However, routine use of these tools is lagging, as evidenced by a recent survey of VHA radiation oncologists. Use of these tools in the clinic will enhance the quality of end of life care and decision making.
Corticosteroids have classically been used in the treatment of brain metastases either alone for supportive care or in combination with RT. Steroids are recommended to provide symptom relief in patients with symptoms related to cerebral edema or mass effect.44 Steroids have been shown to mitigate edema and improve neurologic deficits in about two-thirds of patients with brain metastases.36,45 The effect of corticosteroids is thought to be mediated through inhibition of prostaglandin synthesis, reduction in vascular permeability, and anti-inflammatory properties.46 A common corticosteroid regimen is a 10-mg loading dose of dexamethasone, followed by 16 mg daily in divided doses. For patients without neurologic deficits or cerebral edema, it is reasonable to defer corticosteroid use only when patients are symptomatic.
In general, WBRT is considered an appropriate treatment option for patients with multiple brain metastases based on data suggesting an improvement in OS compared with the use of corticosteroids alone.47 Whole brain radiation has been shown to result in the improvement of baseline neurologic deficits or the prevention of further symptom progression.48 The partial or complete metastasis response rates are on the order of 60%.38 Tumor regression after WBRT has been associated with preservation of neurocognitive function as well as prolonged survival.49
For good prognosis patients with a single brain metastasis and good performance status, the use of surgery or radiosurgery added to WBRT has been associated with improved OS (Table). The RTOG 9508 randomized trial of WBRT with or without SRS demonstrated a survival advantage with SRS, with median survival times of 6.5 months with WBRT + SRS vs 4.9 months with WBRT alone.50 Similarly, a randomized trial evaluating WBRT alone compared with surgery followed by WBRT in patients
with good prognosis demonstrated significantly improved OS in the surgery group (median 40 weeks vs 15 weeks).51 In general, WBRT or postoperative RT to the tumor bed is still indicated after surgical resection, based on randomized data showing a reduction in tumor bed recurrence with postoperative RT.52
For patients with only 1 to 3 brain metastases and a favorable prognosis, surgery and SRS can be considered treatment options, oftentimes with WBRT. The EORTC randomized trial of patients with 1 to 3 brain metastases was designed to determine the benefit of WBRT after treatment with surgery or SRS. In this study, 119 patients underwent SRS and 160 patients underwent surgical resection.53 Both groups of patients were randomized to observation vs adjuvant WBRT. This study demonstrated reduced rates of intracranial relapse with WBRT, however, without any change in OS. Although there is concern that WBRT may impair cognitive function with no clear survival benefit after surgery or SRS, WBRT does reduce recurrence rates in the brain and the need for further treatment.54 Therefore, decisions regarding WBRT in such a setting should be made only after a detailed discussion with a radiation oncologist regarding risks vs benefits of treatment as part of the informed decision-making process.
Conclusions
Palliative RT plays an important role in the management of metastatic cancer to provide symptom relief and is a cost-effective treatment option for bone and brain metastases. Life expectancy and tumor characteristics should be considered when making treatment recommendations to ensure selection of regimens that complement patients’ unique situations. Timely referrals for treatment are important to optimize treatment results.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.