The ‘skinny’ on eosinophilic esophagitis
ABSTRACTEosinophilic esophagitis—a disease that even most physicians know little about—is becoming increasingly common. Often starting in childhood with eating difficulties and symptoms of gastroesophageal reflux disease, it progresses with increasing inflammation, fibrosis, and strictures until the esophagus is visibly narrowed on radiography. Early recognition and treatment with an allergen-avoidance diet and steroids are critical to avoiding or postponing complications.
KEY POINTS
- Eosinophilic esophagitis is an allergy-mediated, systemic disease.
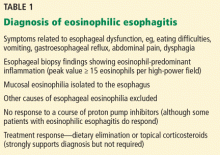
- It is diagnosed by characteristic symptoms, esophageal biopsy (peak value ≥ 15 eosinophils per high-power field), and response to allergen avoidance or treatment with steroids.
- Therapy with a proton pump inhibitor should be tried even for patients with a classic presentation.
- Strict dietary avoidance of allergens has been shown to resolve the disease but is often impractical.
- Dilation is indicated for a narrowed esophagus but must be done cautiously because of the risk of tearing.
- How best to monitor the disease (eg, by annual endoscopy) is still uncertain.
Eosinophilic esophagitis is a new disease defined by specific criteria that include a constellation of symptoms. Consensus guidelines define it as a chronic antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.1
Ten years ago, a biopsy that revealed eosinophils in the esophagus was diagnostic, because normally eosinophils are not seen in the esophagus. The current definition has evolved to become more comprehensive and includes clinical, demographic, and radiographic criteria.
This article presents an overview of eosinophilic esophagitis—its pathogenesis, epidemiology, clinical presentation, diagnosis, and management.
ALLERGIC ORIGIN
Eosinophilic esophagitis is best regarded as a systemic rather than a single-organ disease, although current treatments are mostly directed specifically at esophageal inflammation. Evidence is clear that eosinophilic esophagitis is allergy-mediated.
The current “two-hit” etiologic model involves exposure first to aeroallergens that prime the esophagus, followed by food allergens that cause an eosinophilic response with antigen recognition and stimulation of immune cells from the bone marrow. Other allergic avenues may also be present, including those involved with atopy, asthma, eczema, and food allergies, which stimulate the Th2 pathway and lead to esophageal eosinophilia and inflammation.2
The two-hit model is supported experimentally: the disease can be induced in mice by injecting ovalbumin under the skin as a sensitizing agent, then exposing the airway to an aerosol of Aspergillus fumigatus, producing an allergic reaction involving classic Th2 allergy pathways.3 Further evidence is that many patients report that asthma or rhinitis developed years before esophageal disease began.
Patients with eosinophilic esophagitis and their family members have a high prevalence of allergies, and the disease frequently flares up during allergy season. Endoscopic biopsy specimens from patients often reveal increased T cells, mast cells, interleukin (IL)-5, and tumor necrosis factor alpha, all of which stimulate eotaxin and are essential to an allergic reaction. They also have high levels of CD3, CDA, and CD1A antigen-presenting lymphocytes, which are all associated with allergy.
Eosinophilic esophagitis responds to allergy medications, including corticosteroids and IL-5 or IL-13 mast-cell inhibitors. The strongest evidence for an allergic etiology is that withdrawing culpable food allergens leads to resolution of the disease. Peterson et al4 gave 18 adults with eosinophilic esophagitis an elemental diet (ie, a pure amino acid, carbohydrate-based diet in which all suspected allergens have been removed), and in 2 to 4 weeks, the mean number of eosinophils seen histologically fell from 54 to 10 cells per high-power field. The response was nearly complete (≤ 10 eosinophils per high-power field) in 72% of patients. When patients resumed a normal diet, the eosinophil content increased substantially within a few days.
Role of leaky tight junctions
Normally, the junctions between epithelial cells are tight, but many conditions, including allergic and autoimmune diseases, are now believed to involve altered permeability of this tissue. Tight-junction proteins play an important role in regulating antigen delivery and are modulated by cytokines. Activation of cytokines causes the membrane to become more permeable, allowing antigens to get through, leading to an enhanced reaction. In eosinophilic esophagitis, it is postulated that food antigens that pass through the leaky membrane activate CD1-antigen-presenting cells, which then initiate an allergic reaction.5–9
PREVALENCE IS INCREASING
Eosinophilic esophagitis was first described in 1993 with a report of 12 patients who had dysphagia, normal endoscopy, no acid reflux, and intraepithelial eosinophilia.10 The authors recognized that these patients had a distinct disease.
Since then, the disease has increased in prevalence. Kapel et al11 reviewed more than 74,000 endoscopy slides from a national pathology database and found 363 cases, with increasing prevalence during the study period from 2002 to 2005. Looking back further in a similar study, Whitney-Miller et al12 found a 0.3% prevalence from the years 1992 to 2000 vs 3.8% from 2001 to 2004.
Sealock et al13 reviewed the literature to assess the prevalence of eosinophilic esophagitis and found considerable variation depending on the populations sampled. One study from Sweden14 found a prevalence of 0.4% by performing endoscopy in 1,000 randomly selected people from nearly 3,000 responders to a questionnaire on abdominal symptoms. A study based on a Swiss database15 found only a 0.02% prevalence. Other studies show higher rates: a study from Florida that examined biopsy specimens from patients who underwent endoscopy for any reason found a prevalence of 1%.16 Another US study found a 15% prevalence in patients with dysphagia.17 Since these studies were done nearly a decade ago, we can expect the prevalence to be higher today.
Celiac disease has also been increasing in recent decades, as has gluten sensitivity. Allergies in general are on the rise worldwide, including asthma and atopic dermatitis. Theories as to the cause of these increases have focused on ambient antigens, food additives, proton pump inhibitors (PPIs), and the microbiome.18,19
DIAGNOSING EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis is diagnosed with a combination of symptomatic, histologic, and radiographic findings (Table 1). The classic patient is a white male—a child, teenager, or young adult—with dysphagia.
A case series of 23 adult patients20 found a mean age of 35 (age range 18 to 57), with a male preponderance (14:9). There is commonly a history of other allergies, including asthma, allergic rhinitis, and atopic dermatitis. Patients more commonly present with dysphagia than heartburn or other esophageal symptoms.11
Endoscopic findings—eosinophils, later fibrosis
Finding eosinophils in the esophagus is nonspecific and is not sufficient to make the diagnosis. Other systemic diseases can involve esophageal eosinophilia, including Churg-Strauss syndrome, Crohn disease, and helminthic diseases. Whether some are related to eosinophilic esophagitis or are independent is not well understood.
Characteristic findings on endoscopy include a corrugated or ringed appearance and linear furrows, resulting from fibrosis and scarring. “Micro-tears” may also be visible projecting linearly up the esophagus. Multiple white specks are signs of conglomerations of eosinophils and are easily confused with yeast infection. Strictures from scar tissue cause the mucosa to be tight and fragile, making the esophagus very susceptible to tearing during endoscopy.
After years of untreated disease, the esophagus becomes increasingly inflamed and fibrotic. Adult patients with eosinophilic esophagitis who were followed for a decade were found to develop increasing collagen deposition in which the submucosa or even the entire esophageal wall was diffusely fibrotic.21
Radiographic findings—a narrow esophagus
On radiography, the esophagus may appear narrow—not uncommonly one-third to one-quarter the caliber of a normal esophagus. As the esophagus progressively narrows, both eating and treatment become extremely difficult.
Symptoms are different in children and adults
Symptoms reflect the endoscopic changes over time. In children, the condition manifests with feeding difficulties, vomiting, symptoms of gastroesophageal reflux, and abdominal pain as signs of inflammation. As the esophagus becomes fibrotic, teenagers and young adults tend to present with strictures, dysphagia, and food impaction. Of patients who present to an emergency department with food impaction, the major cause is now eosinophilic esophagitis.22
It is important to pay attention to symptoms in children to diagnose the condition and start treatment early to prevent or postpone disease advancement. Medical therapy does not clearly reverse the fibrosis.
As in many chronic benign diseases, patients learn to compensate, so a careful history is essential. Many deny having a swallowing problem, but questioning may reveal that they have always been slow, picky eaters, consuming mostly soft foods and drinking fluids with every bite.