Stereotactic body radiotherapy for stage I non–small cell lung cancer
ABSTRACTSurgical resection for patients with stage I non–small cell lung cancer (NSCLC) produces high long-term survival rates, but many patients are ineligible for surgery because of medical comorbidity or other factors. Stereotactic body radiotherapy (SBRT) is the standard of care for patients with medically inoperable stage I NSCLC. Studies have reported local control rates with SBRT of about 95% when an adequate radiation dose is used. Lymph node failure averages less than 5%, while distant metastatic recurrence represents the most common site of failure. SBRT is generally safe and well tolerated even by patients with substantial pulmonary comorbidities. On average, lung function tests reveal little or no change from baseline, although individual patients may exhibit changes in pulmonary function after treatment. Most studies report pneumonitis rates of 0% to 5%. Ongoing clinical trials are investigating single-fraction SBRT and evaluating the maximal tolerated dose for centrally located tumors.
Surgical resection for patients with stage I non–small cell lung cancer (NSCLC) is typically associated with survival rates of 60% to 70% after 5 years, and as high as 80% in some series.1 Although lobectomy or pneumonectomy improves outcomes compared with sublobar resection for many patients, a substantial number are ineligible for standard surgical resection because of cardiovascular disease or other conditions that are associated with unacceptably high perioperative risk. Observation alone is not a good strategy for patients who are ineligible for surgery. Studies comparing treatment outcomes associated with resection, radiation, and observation have demonstrated much shorter survival times and higher mortality for patients treated with observation only.2
Stereotactic body radiotherapy (SBRT) is the new standard of care for patients with medically inoperable stage I NSCLC. SBRT differs from standard radiation therapy in terms of dose, fractionation, field size, and targeting. Compared with standard radiation, SBRT offers a shorter and more convenient treatment regimen with improved local control and survival while lowering treatment cost.3,4 Although cancer-specific outcomes of patients in SBRT series are similar to those in surgical groups, they are not truly comparable because of dissimilarities between the two populations. The inoperable group has higher rates of comorbidity and death compared with the medically operable group; as many as one-third die from comorbid conditions rather than cancer, leading to short follow-up in many SBRT series. Surgical resection remains the standard of care for operable stage I NSCLC.
STEREOTACTIC RADIATION FOR PATIENTS WITH INOPERABLE LUNG CANCER
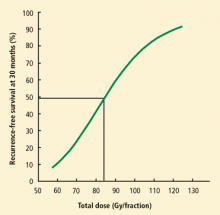
Modern standard external beam radiation doses without chemotherapy for stage I lung cancer are approximately 60 to 74 Gy. The dose fractionation schedule used with SBRT delivers much higher equivalent doses (83 Gy to 150 Gy), although the true biologically equivalent dose (BED) is not yet perfectly understood.8 Most clinical studies that have examined the effectiveness of SBRT have demonstrated local control rates in excess of 90% to 95% when an adequate dose (BED ≥ 100 Gy) is utilized, since the dose-response curve appears to plateau at this level.9 These response rates are higher than the 50% to 60% rate observed with conventional radiation.3,4 Efforts to confirm these comparative results in randomized trials have been largely abandoned because of the perceived advantage with SBRT.
PERIPHERAL VERSUS CENTRAL TUMORS
Stereotactic body radiotherapy has been referred to as “radiosurgery,” in part because the extremely high doses used to treat tumor are ablative to the immediate surrounding tissue. The consequences of ablation depend on whether the treatment involves parallel or serial tissue. Parallel tissue, such as lung, kidney, or liver, remains functional after the ablation or removal of small subunits if adequate volume of functional organ remains. With serial tissue such as the spinal cord or bowel, damage to one section results in loss of function at distal sites. Although the lung is parallel tissue, it includes serial structures such as the trachea and proximal bronchial tree. Tumors located within 2 cm of the proximal bronchial tree are classified as central, whereas tumors outside this zone are peripheral.
Peripheral tumors
Peripheral lung tumors are surrounded by only parallel tissue, and no maximum point-dose limit has been identified for their treatment. A recent cooperative group study (Radiation Therapy Oncology Group [RTOG] 0236) enrolled 55 patients, 80% with tumor stage IA (T1 N0) and 20% with stage IB (T2 N0).10 Patients with bronchoalveolar histology were excluded from the study. Patients received three radiation treatments of 20 Gy each (BED of 180 Gy) to their known tumor with a small margin, and were followed with serial computed tomography (CT). After a median follow-up of 34 months, only one of the 55 evaluable patients had a local tumor failure, for a local control rate of 97.6%. Three patients had recurrences in the initially involved lobe for a 3-year local control rate of 90.6%; two patients had nodal failures for a 3-year local regional control rate of 87.2%; and 11 patients had disseminated recurrences, for a 3-year distant failure rate of 22.1%.
Survival after 3 years was approximately 50%, which is much better than the survival rate typically attained with standard radiation therapy. Further, only 10 of the 26 deaths were attributed to lung cancer while 16 patients died of comorbid conditions such as stroke or myocardial infarction, illustrating the difficulty in tracking overall survival as a measure of efficacy in this medically fragile population.
Adverse events in this study were relatively rare. Seven patients had grade 3 or higher pulmonary complications, including hypoxia, pneumonitis, and pulmonary function test changes. Of note, the study scored changes in pulmonary function as toxicity; however, in this population, where nearly all patients have underlying lung disease, chronic obstructive pulmonary disease (COPD) exacerbations are also common.
Our own analysis of pulmonary function changes in patients treated with SBRT at Cleveland Clinic demonstrated that while there was no significant change in average baseline, pulmonary function in almost 10% of patients met criteria for a grade 3 pulmonary toxicity. A similar number of patients had a proportional improvement in pulmonary function, however. Given a nearly comparable distribution of pulmonary function changes in both directions with no significant deviation from baseline in aggregate, most of these fluctuations may be related to changes in the patient’s underlying comorbidities rather than effects of treatment.
RTOG 0236 demonstrated an excellent level of local control (97.6%) using 3 fractions of 20 Gy each (BED 180 Gy total). As noted, the dose response may plateau at 100 Gy BED,9 which raises the question of whether the radiation dose levels used in this study were higher than necessary. A recently completed randomized phase 2 clinical trial conducted by the RTOG compared 34 Gy in a single fraction versus 48 Gy in 4 fractions, and a similar study by Roswell Park Cancer Institute, Buffalo, New York, and Cleveland Clinic is comparing 60 Gy in 3 fractions versus 30 Gy in a single fraction. These studies, once mature, should help define the optimal radiation dose and treatment schedule for patients with inoperable peripheral tumors.
Central tumors
Centrally located tumors are in proximity to both parallel tissues (normal lung) and serial tissues (trachea, bronchial tree, or esophagus), as well as imperfectly categorized tissues (heart and great vessels). An important question is whether it is possible to reach a radiation dose level of 100 Gy BED or higher in these tumors without causing excessive toxicity to normal tissues. Although there is a potential risk of cardiotoxicity with chest radiotherapy, clinical studies of SBRT for lung cancer have not demonstrated any evidence of toxicity to the heart or the great vessels with focal radiation. Some studies have suggested that radiotherapy of central lung tumors may be associated with other adverse events.
Awareness of central versus peripheral tumor locations was first raised in an early phase 2 study in which patients were treated with 60 to 66 Gy in 3 fractions over a period of 1 to 2 weeks. Grade 3 or higher toxicity during 2 years of follow-up was noted for 46% of patients with central tumors and 17% of patients with peripheral tumors.11 Six deaths that occurred during the study were considered to be possibly treatment-related, including four cases of bacterial pneumonia, one patient with pericardial effusion, and one patient with hemoptysis that was later ascribed to carinal recurrence.
Other studies using lower fraction sizes, however, have demonstrated excellent efficacy and safety in treating central tumors with SBRT. In early Japanese studies12,13 that used smaller fractions without tissue constraints, no differences in toxicity were noted with treatment of central versus peripheral tumors. A European study similarly demonstrated more than 90% local control at 3 years for a regimen of 60 Gy in 8 fractions (7.5 Gy/fraction).14 Currently the RTOG is conducting a dose escalation study examining doses from 50 Gy to 60 Gy (10 Gy to 12 Gy per fraction in 5 fractions). The study has reached its highest level (60 Gy in 5 fractions) with no evidence of excessive toxicity reported.