Deep brain stimulation for movement disorders: Patient selection and technical options
ABSTRACTDeep brain stimulation (DBS) is used as a treatment for movement disorders. Unlike ablative procedures, DBS is reversible and adjustable. It is approved in the United States for treatment of Parkinson disease (PD), dystonia, and tremor. This surgical procedure is considered safe and effective for the management of the motor symptoms of these disorders, although it does not cure the underlying conditions. Potential complications of DBS surgery include intracranial hemorrhage, infections, and complications related to the hardware. There may also be complications related to stimulation or programming, although these are usually associated with dosages of dopaminergic medications and are reversible. DBS is usually performed under conscious sedation with awake evaluation during intraoperative physiologic testing. Typically, the procedure is performed with stereotactic image guidance, using computed tomography or magnetic resonance imaging (MRI) for targeting. Surgery can be accomplished with stereotactic frames or frameless systems. Recently, intraoperative MRI guidance has become available and is an alternative to the traditional surgical procedure, allowing for implantation of the DBS device under general anesthesia.
Implantation of a deep brain stimulator is the most common surgical procedure performed in the United States and industrialized world for the management of advanced movement disorders. These procedures are US Food and Drug Administration (FDA)–approved for the management of the symptoms of Parkinson disease (PD) and essential tremor. Deep brain stimulation (DBS) is also approved for managing primary generalized dystonia and torticollis under a humanitarian device exemption.
Deep brain stimulation has largely replaced ablative procedures such as thalamotomy and pallidotomy. While ablative procedures can be effective for the symptoms of movement disorders, they cause a permanent lesion in the targeted nuclei and are therefore not reversible. DBS is considered safer because it can be adjusted over time and the location of the leads can be revised.1 On the other hand, regular maintenance of implanted hardware may be considered a disadvantage of DBS.
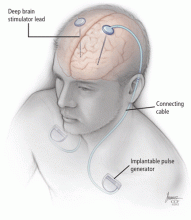
HARDWARE AND TARGETS
While ablative procedures do not require implantable hardware, DBS consists of permanently implanted neurostimulation systems. The battery-powered pulse generators typically last for several years but require multiple replacements during a lifetime. In addition, if other hardware components fail, surgical revision may be required to maintain treatment efficacy. Surgery involving implantation of hardware carries a higher risk of infection than does a nonimplantation procedure. If infections occur, removal of the hardware is often required, with reimplantation performed after the infection clears. In addition, the expense of DBS hardware may limit availability in some cases.
Three components
Target nuclei
Several nodes or nuclei can serve as targets for DBS. In patients with PD, the most common surgical target is the subthalamic nucleus (STN), either unilaterally or bilaterally.2 The globus pallidus pars interna (GPi) is also a viable target and is preferred for some patients with PD. The most common target for managing essential tremor is the ventral intermediate nucleus (VIM) of the thalamus, which can also be the target of choice for patients with tremor-predominant PD. However, the GPi and STN are usually preferred over the VIM in patients with PD because stimulation of these targets can relieve symptoms other than tremor, such as rigidity and bradykinesia. Bilateral stimulation of the GPi is the most frequent approach in patients with generalized torsion dystonia and torticollis, although the STN and thalamic nuclei (off-label) are also considered options.
PATIENT SELECTION
Patients are evaluated in our center at Cleveland Clinic by a multidisciplinary team that includes a movement disorder neurologist, a subspecialized neurosurgeon, a movement disorder neuropsychologist, and a psychiatrist with special interest in the behavioral comorbidities of movement disorders.3 Neuroimaging is included in this assessment. We have also included physical therapy as part of the initial evaluation in order to gain insight into the patient’s limitations and develop rehabilitation strategies that may enhance the outcomes of surgery or provide alternatives should surgery not be indicated. This evaluation provides extensive data that are then reviewed by the team in a conference dedicated to discussing candidacy for DBS or options for managing the symptoms of advanced movement disorders. Behavioral and cognitive issues are assessed in detail and, in our experience, are the most common reasons for not recommending DBS.
An important part of the evaluation of patients with PD is a formal test with rating of the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS) with the patient off medications for 8 to 12 hours and then after a test dose of levodopa. At our center, this off/on test is videotaped so that the responsiveness of individual symptoms to levodopa can be reviewed later in conference.
Risk of cognitive decline
While DBS is considered safe and effective, there is a risk of cognitive decline in some patients. In most patients, long-term stimulation-related cognitive decline may be detected with formal measures but is not clinically significant and is outweighed by the motor and quality-of-life benefits of surgery. In some patients, long-term cognitive decline can be significant and can limit function. Cognitive neuropsychologic testing provides valuable information in this regard. Patients with preserved cognitive function seldom experience significant decline with DBS while those with substantial baseline impairment are thought to be at greater risk. Patients who meet criteria for dementia are usually not considered candidates for DBS, but exceptions exist. Transient perioperative cognitive difficulties are more common than persistent deficits, and typically resolve within a few weeks (see “Complications of deep brain stimulation”).
Benefits in Parkinson disease
Deep brain stimulation can address several symptoms of PD but with varying effects. Tremor, rigidity, and bradykinesia usually improve substantially. Gait has a more variable response, and balance is typically refractory. A general rule is that symptoms that improve with a single dose of levodopa should also improve with DBS. (Tremor, however, will most often respond to DBS even if refractory to medication.) Good candidates for surgery typically have a greater than 30% improvement in UPDRS motor score with levodopa challenge, but sometimes, improvement in the total score is less informative than evaluation of the effects of levodopa on particular symptoms. Treatment effects can be compared with the patient’s expectations for surgery in order to infer whether the goals for symptom improvement are realistic.