Necrotizing pancreatitis: Diagnose, treat, consult
ABSTRACT
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists and general internists are on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care.
KEY POINTS
- Selective and appropriate timing of radiologic imaging is vital in managing necrotizing pancreatitis. Protocols are valuable tools.
- While the primary indication for debridement and drainage in necrotizing pancreatitis is infection, other indications are symptomatic walled-off pancreatic necrosis, intractable abdominal pain, bowel obstruction, and failure to thrive.
- Open surgical necrosectomy remains an important treatment for infected pancreatic necrosis or intractable symptoms.
- A “step-up” approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
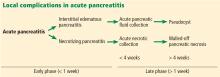
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
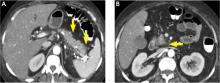
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
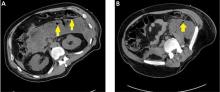
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.